
New Catalyst set to reshape Hydrogen Production
RESEARCHENERGYNANOMATERIALS

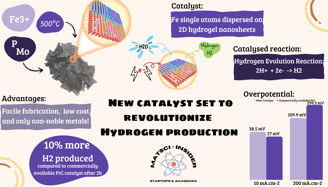
City University of Hong Kong (CityU) has made a huge leap forward in the world of hydrogen production with their latest discovery! This novel catalyst, based on Iron single atoms dispersed on a mineral gel, is set to revolutionize the way we produce clean and renewable energy. Initial tests have shown incredible potential, and we can't wait to see where this technology takes us!
The race for green Hydrogen is still on, and this last breakthrough by Professor Lu Jian and his team might actually make a difference!
A bit of context:
If you did not follow up until now, quick summary of the situation: Green hydrogen is produced through a process called electrolysis, which uses electricity to split water molecules into hydrogen and oxygen (Hydrogen Evolution Reaction: 2H⁺ + 2e⁻ → H₂). The electricity used in this process can come from renewable energy sources, such as wind or solar power, which means that the hydrogen produced is "green" and doesn't produce any pollution or greenhouse gases. We can then store this hydrogen and use it later to produce electricity using the reverse reaction (Hydrogen Oxidation Reaction: H₂ → 2H⁺ + 2e⁻).
Quite simple right? Well, yes... and no. There is a reason why 95% of the hydrogen we produce today comes from fossil fuels and not electrolysis: Electrolysis is not efficient enough. Producing Hydrogen using Natural gas or coal costs an average of 1 to $2 per kg of H₂ produced, whereas it costs more than $5 to produce a kg of H₂ by electrolysis (Muhammet Kayfeci, Solar Hydrogen Production, 2019). To improve this, a race started a few years ago to improve the efficiency of electrolysis.
Improving efficiency, what does it mean? The process must be efficient in both ways. In one direction, we want to improve the yield of H₂ produced for a given amount of current, and in the other direction, we want to retrieve as much electricity as possible from the stored H₂.
How do we do it? There are many parameters to take into consideration, but currently, most of the research is focusing on improving the catalyst. A catalyst reduces the amount of energy required to drive the reaction. It works by providing an alternative reaction pathway that is energetically favourable, allowing the water-splitting reaction to occur at a lower voltage. Currently, the best catalysts we produce are made of precious metals, especially Platinum. But keep in mind that, in order for a catalyst to make its way out of the lab, it needs to be as cheap and easy to produce as possible.
Is this the breakthrough we were waiting for?
In this race to lower the content of noble metals, CityU is taking a drastically different approach. No noble metals in sight here, the team has developed a highly efficient Iron single-atom electrocatalyst that uses mineral hydrogel nanosheets as a precursor.
The synthesis method is quick and straightforward, the first step is done at room temperature and is followed by a treatment at 500°C in a second step. This makes this process much more convenient and economical than previous methods that require high temperature, high pressure, and long periods of time for self-assembly of single-atom precursors.
The experiments found that the new catalyst has excellent electrocatalytic activity and long-term durability in the hydrogen evolution reaction, with an overpotential of only 38.5 mV (20-40mV for the best Pt Catalyst!) and ultra-stability with no degradation in performance over 600 hours (for more detailed information you can access the full paper here).
According to Professor Lu, this is one of the best performances achieved by non-noble-metal HER electrocatalysts. The use of mineral gel to synthesize monatomic dispersed heterogeneous catalysts provides a promising approach for scalable production of cheap and efficient catalysts, which could help reduce the cost of hydrogen production in the long term.
From the lab … to the market?
One question remains: is this paper going to have an impact on the Green Hydrogen market, or will it just be one more discovery that won’t ever leave academia? Only the future will tell, but we can make an educated guess.
Around the world, the urge for low-carbon alternatives to fossil fuels is getting strong. Hydrogen, as a way to store unpredictable renewable energies, is one of the most important technology to harness and government funding for projects in this area are flourishing around the world. We can cite the Office of Clean Energy Demonstrations in the US which have $8 billion in funding to support the development of clean hydrogen hubs to improve clean hydrogen production, processing, delivery, storage, and end use; or the EU hydrogen strategy in Europe with ambitious goals to scale up Hydrogen production before 2030.
This paper is published at a pivotal moment and might benefit from it. No doubt that this new cheaper method will at least be tried by different actors and if it proves to be easily scalable it might be implemented on a larger scale. Nevertheless, there is also a risk that, just like with the race for nuclear energy a few decades ago, one method will be developed and mastered while the others remain prototypes and lab curiosities. This happened with exciting technologies like molten salt reactors that showed promising yields but suffered from the snowball effect following the widespread use of light-water pressurized reactors.
To summarize, we have here an exciting new catalyst that has the potential to solve our current issue with green hydrogen production. The low-cost, facile fabrication and good performance of this catalyst make it a strong candidate to be used outside of academia, and it could potentially hit the market relatively soon. Now we just have to wait and see!




